Why share your data using Vivli?
• Ease of sharing – Vivli makes it easy for you to meet your data sharing commitments to funders as well as journals
and above all to the trial participants.
• A process flexible to meet your needs – Vivli’s process allows you to set key parameters for how your data is shared;
the Vivli team will then manage the actual sharing for you.
• Metrics report of data use – Vivli will provide you with a report detailing how your data has been used in secondary
publications – data you may use in future grant applications.
Key decisions to make about how you share your clinical research data
- Expedited Review or Independent Review? The Vivli team may conduct an expedited review process for requests for
your data or the Independent Review Panel may review these proposals on your behalf. - Will you/your institution anonymize your data or do you need help to anonymize? Vivli only accepts the contribution
of anonymized data. Your institution may provide support or Vivli has anonymization vendors who will offer support
for this service. This cost may be an allowable expense under your existing grant. If you have questions, please reach
out and we can discussions options. - Are you ready to share now or do you require an embargo period? Most researchers are ready to share when they
contact us; however, we do allow for an embargo period of 6 months from when your data is uploaded to our platform
if necessary. - Data downloadable or in a secure research environment? The default option is for us to securely store your data and
to make it available upon request via download for subsequent research. If a study is required to be shared via a secure research environment, please contact us.
How much does it cost to share and archive my data long-term on the Vivli platform?
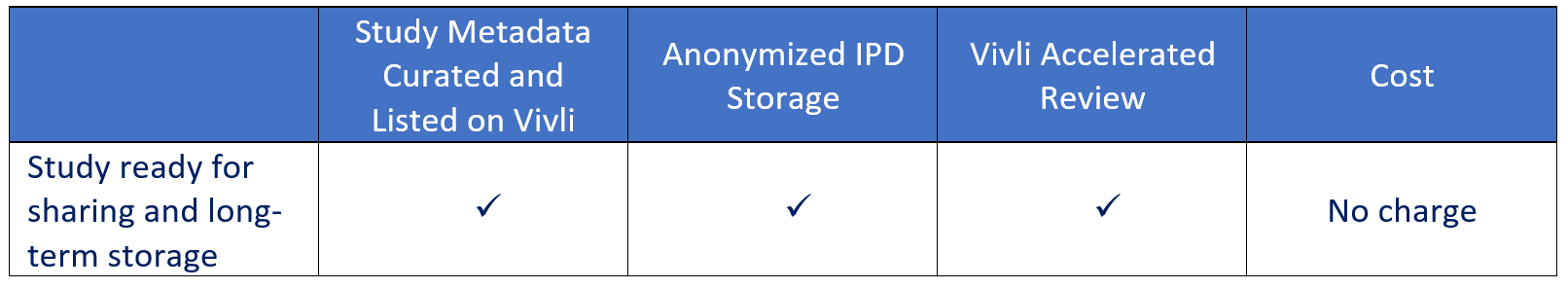
Additional options may incur costs, reach out to support@vivli.org with questions.
Steps to sharing your clinical research data on Vivli
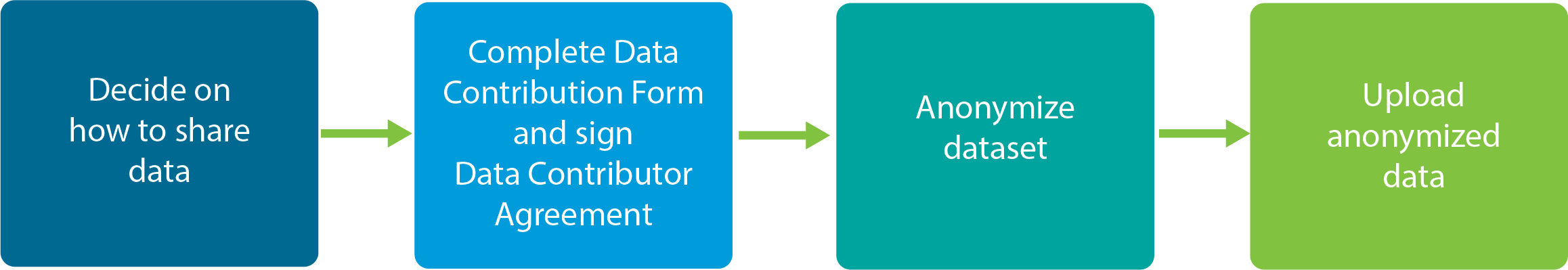
1. Decide on how you want to share your data from your clinical research. See key decisions to make
about sharing your clinical research data.
2. Complete and sign Data Contribution Form and the Data Contributor Agreement (DCA). The DCA needs to be read,
understood and signed by the Principal Investigator and an institution official. If you don’t know who your
institution official is, in most organizations a good place to start is the Grants and Contract office. See this template
email to send to this office.
3. Once the forms are completed, Vivli will reach out with next steps about how to upload your anonymized data.
What do I need to share as part of my data package?
Data package – Protocol, anonymized individual participant-level dataset, data dictionary, and statistical analysis plan are recommended; additional elements such as an annotated case report forms, clinical study report, and analysis ready datasets can also be included.

