Leading Institutions Sign On to International Platform Which Aims to Accelerate Medical Research
Washington, DC—Today, the nonprofit organization Vivli launched a novel data-sharing and analytics platform to help researchers move faster toward new treatments and cures. This innovative platform makes it easy for researchers worldwide to discover, share and analyze data from clinical trials, regardless of who sponsored the research or where the research took place. Vivli launches today with 2,500 studies from 15 organizations with data from more than 98 countries across numerous disease areas, including diabetes, Alzheimer’s and malaria, and representing the contributions of more than 1.3 million trial participants.
The Vivli platform launches at a crucial time in the evolution of science. Increasingly, researchers are being required to make their data openly available. New guidelines issued recently by the International Committee of Medical Journal Editors demand that researchers share the data underlying their published results. Government and non-profit research funders are stipulating data-sharing as a condition of their grants. The culture within industry is also shifting to reward more open sharing of data. The Vivli platform boosts these emergent data-sharing efforts by bringing data from all clinical trials together in one place.
“To this day, much of the world’s clinical trial data is not shared, and even when it is shared, it is not integrated across the entire clinical research ecosystem,” said Vivli’s Executive Director Rebecca Li, PhD. “Vivli acts as a bridge, enabling researchers across the world to build upon each other’s work and speed up science.”
Vivli provides researchers with a secure, easy-to-use online platform to store and share clinical trial data across all diseases, nations and research entities. Vivli also allows researchers to freely combine and analyze data from multiple trials using state of the art tools. This means researchers can validate research findings—from confirming a treatment’s safety to identifying side effects—and avoid duplicative studies, thus reducing costs and shielding research participants from unnecessary risks. The data can also be combined to ask new research questions, such as how a disease evolves over time, and gain information to inform the design of future studies.
To ensure sustainability of the platform, Vivli is supported by a robust membership structure for industry, academic and non-profit research entities and their funders. Members commit to contribute data and, in the case of philanthropic organizations, cover the costs for grantees to share their data on Vivli. Current members and those with data contributing agreements include AbbVie, Aegerion Pharmaceuticals, Inc., Biogen, Inc., BioLINCC (National Heart, Lung, and Blood Institute), Critical Path Institute, Duke University, GlaxoSmithKline, plc, Harvard University; ImmPort (National Institutes of Health), Johns Hopkins University, Pfizer, Inc., Project Data Sphere, LLC, Takeda, The Leona M. and Harry B. Helmsley Charitable Trust, and the University of California, San Francisco.
Vivli evolved from a project at a policy center, The Multi-Regional Clinical Trials Center of Brigham and Women’s Hospital and Harvard University, to enhance access to clinical trials data. For five years, a group of volunteers embarked on a mission to define, design and launch a global solution for sharing clinical trial data. “Sharing clinical trial data is critical in order to move science forward. We owe nothing less to the people who put themselves at risk by participating in these studies that benefit us all,” said Li.
The Vivli platform was built with grants from the Doris Duke Charitable Foundation, the Laura and John Arnold Foundation and The Leona M. and Harry B. Helmsley Charitable Trust. The platform is powered by Microsoft.
Statements from Vivli Supporters
Takeda
“Takeda is strongly committed to our core value of integrity and an important way to demonstrate this commitment is through maximizing the transparency of our clinical research. We see enormous potential in Vivli’s vision to create a more connected and interoperable data sharing landscape to positively impact the health of our patients. Takeda is proud to have participated in the development of the Vivli platform from its inception and we are excited to work towards full membership and collaborate with the Vivli community.” – Patrick Cullinan, Head of Clinical Transparency and Science Advocacy, Takeda
Helmsley Charitable Trust
“Every day, we are reminded of the urgent need to accelerate medical research. Too many people around the world are suffering from disease and need more effective treatments,” said Stephanie Cuskley, CEO of the Helmsley Charitable Trust, a founding member of Vivli. “Partners like Vivli offer a unique opportunity for research institutions to maximize their impact by sharing data and working together to propel new scientific discoveries forward.”
Project Data Sphere, LLC
“Aggregated patient-level cancer clinical trial datasets from across industry and academia, and managed within the Project Data Sphere cancer research platform, are already yielding new insights into understanding cancer and improving outcomes for cancer patients,” said Dave Handelsman, Vice President for Project Data Sphere, LLC. “It’s exciting to collaborate with Vivli as their vision of data sharing enables research across a broad range of diseases”.
Duke University
“Duke University School of Medicine is pleased to partner with the Vivli Organization to foster the principles of open science and data access from clinical studies,” said Adrian Hernandez, MD, Vice Dean for Clinical Research, Duke University School of Medicine. At Duke, we are committed to developing research polices, platforms and methods to provide appropriate access to research information, with the ultimate goal of expediting the development of new treatments to improve the health of patients around the world.”
The Bill & Melinda Gates Foundation
“We believe that the tools and discoveries capable of helping us tackle the world’s most pressing challenges – from age-old diseases like malaria to newer threats like drug-resistant superbugs – can only be realized through innovative thinking and global collaboration. Open access to data is critical for both of these things. This is why we are committed to increasing the impact of the research we fund so that it is open and accessible to all through platforms like the one developed by Vivli.” – Steven Kern, Ph.D., Deputy Director, Quantitative Sciences, The Bill & Melinda Gates Foundation
Pfizer, Inc.
“Pfizer participates in Vivli and other data-sharing platforms and collaborations because we believe that providing access to clinical data, when managed responsibly and in ways that respect patients’ privacy and preferences, may advance scientific knowledge and public health,” said Freda Lewis-Hall, M.D., Executive Vice President and Chief Medical Officer, Pfizer. “We hope that the Vivli platform will help the research community to solve unanswered questions for the benefit of patients.”
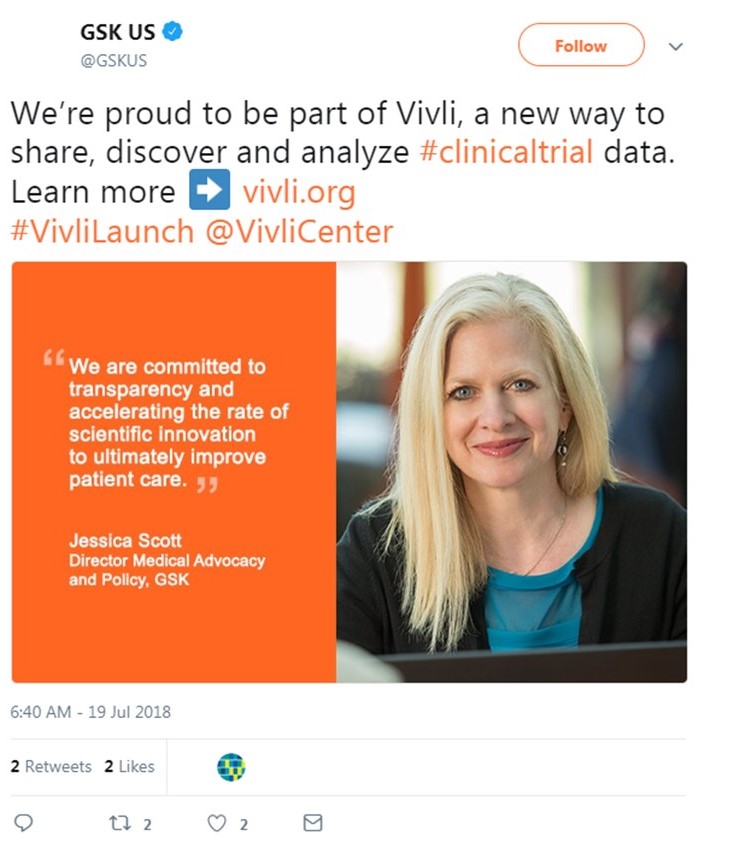
GSK
“We began our journey sharing data with researchers in 2013 with a vision of a global data sharing platform, a one-stop shop where researchers could access data across industry, academic and government funded trials,” said Jessica Scott, Director Medical Advocacy and Policy at GlaxoSmithKline. “We are committed to transparency and accelerating the rate of scientific innovation to ultimately improve patient care. We are pleased to be contributing our trials to Vivli and we look forward to greater data sharing and use of these data across the clinical research enterprise.”
Microsoft
“Strategic partnerships in healthcare and life sciences have become a major focus for Microsoft as an organization. We have a series of initiatives underway that will lead to improvements in the clinical trials process, and an increase of the value that can be extracted from clinical trials data. Vivli’s commitment to the ethical sharing of data for the greater public good and the advancement of open science makes them an ideal partner for Microsoft, and we look forward to deepening the partnership in coming years, as we look for innovative new ways to enhance the Vivli platform.” – Paul Slater, Co-Founder, Life Sciences Innovation, Microsoft
Harvard University
“The mission of Vivli is fully aligned with that of Harvard University in promoting collaborative research and data sharing and transparency,” says Ara Tahmassian, Ph.D., Chief Research Compliance Officer, Harvard University. Our faculty has a long history of data sharing. Our commitment to Vivli reflects our support by providing one platform for our researchers to fulfill ICMJE and funder data sharing requirements.”
About Vivli
Vivli is a non-profit organization working to advance human health through the insights and discoveries gained by sharing and analyzing data. It is home to an independent global data-sharing and analytics platform which serves all elements of the international research community. The platform includes a data repository, in-depth search engine and cloud-based analytics, and harmonizes governance, policy and processes to make sharing data easier. Vivli acts as a neutral broker between data contributor and data user and the wider data sharing community. For more information, visit www.vivliorg.kinsta.cloud and follow us on Twitter @VivliCenter.
 Dr. Li provides a human dimension of the Vivli project: “The fact is, that patients who sign on for these clinical trials, want to share their data. When we talk to patients, they are surprised if their data is not being shared. They want to know that their contribution is playing a part in improving research, and advancing health. Therefore, the data needs to be anonymized to respect the patient’s contribution.”
Dr. Li provides a human dimension of the Vivli project: “The fact is, that patients who sign on for these clinical trials, want to share their data. When we talk to patients, they are surprised if their data is not being shared. They want to know that their contribution is playing a part in improving research, and advancing health. Therefore, the data needs to be anonymized to respect the patient’s contribution.”


 Vivli is an innovative new clinical research data sharing platform with a robust search engine that has been created to meet the needs of researchers worldwide who use clinical research data. Using the Vivli platform, researchers can access anonymized data from more than 7,000 completed clinical trials.
Vivli is an innovative new clinical research data sharing platform with a robust search engine that has been created to meet the needs of researchers worldwide who use clinical research data. Using the Vivli platform, researchers can access anonymized data from more than 7,000 completed clinical trials.