Dear Friends,
2020 has been an unprecedented year for us all. For Vivli, the pandemic has magnified the necessity for data sharing. We’re seeing now, more than ever, that sharing the individual participant-level data that underpins trial results is vital to driving forward both new science as well as scientific consensus.
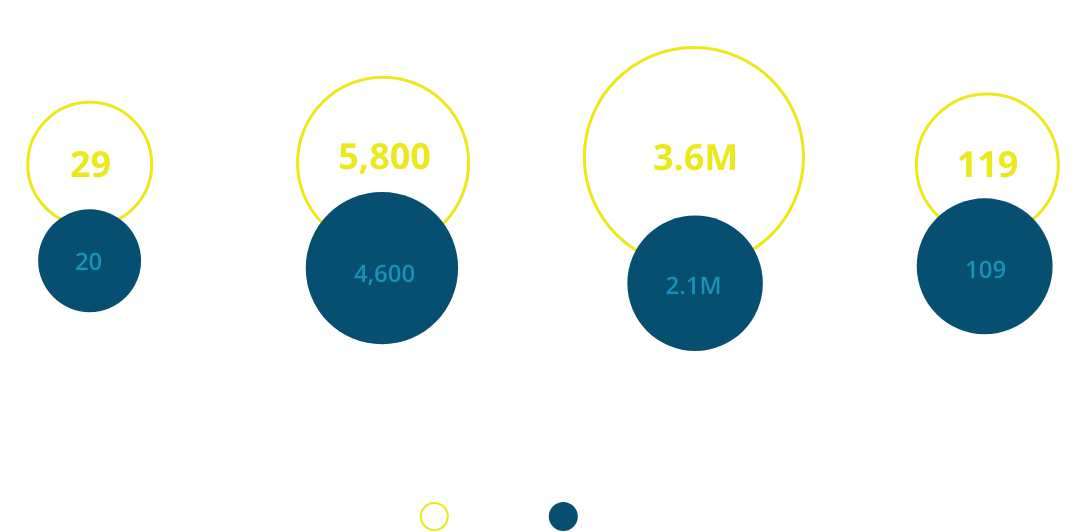
This year, we were delighted to welcome several new members to the Vivli platform, including AstraZeneca, CureDuchenne, Lundbeck, Mitsubishi Tanabe Pharma, Regeneron, Roche, Taiho and UCB. We were also thrilled to see the first wave of secondary research conducted through Vivli come to fruition, with findings already impacting the way clinical trials are run and illuminating new directions for patient care.
As we look ahead, we anticipate Vivli researchers will help to close vital gaps in our understanding not only for COVID-19 but in other diseases represented in the 18 therapeutic areas covered by the Vivli platform. We are enthusiastic to see the results of the hundreds of active proposals that are reusing clinical trial data shared on Vivli–building on prior knowledge to accelerate scientific progress.
To further these efforts, in 2021, we will be focused on:
- Building our list of available COVID data
- Expanding our training resources library
- Increasing partnerships and collaborations with foundations, journals and academics
I would like to thank our members, stakeholders, funders and researchers for their trust and continued support.
 REBECCA LI,
REBECCA LI,
EXECUTIVE DIRECTOR, VIVLI