Learn how the Vivli repository is making it easier for scientists to share and access data, and how you can comply with the NIH’s data management and sharing policy (DMSP) to maximize the value of your research
The National Institutes of Health (NIH) has a policy in place to ensure that data generated by NIH-funded research is accessible to the scientific community starting on January 25, 2023.
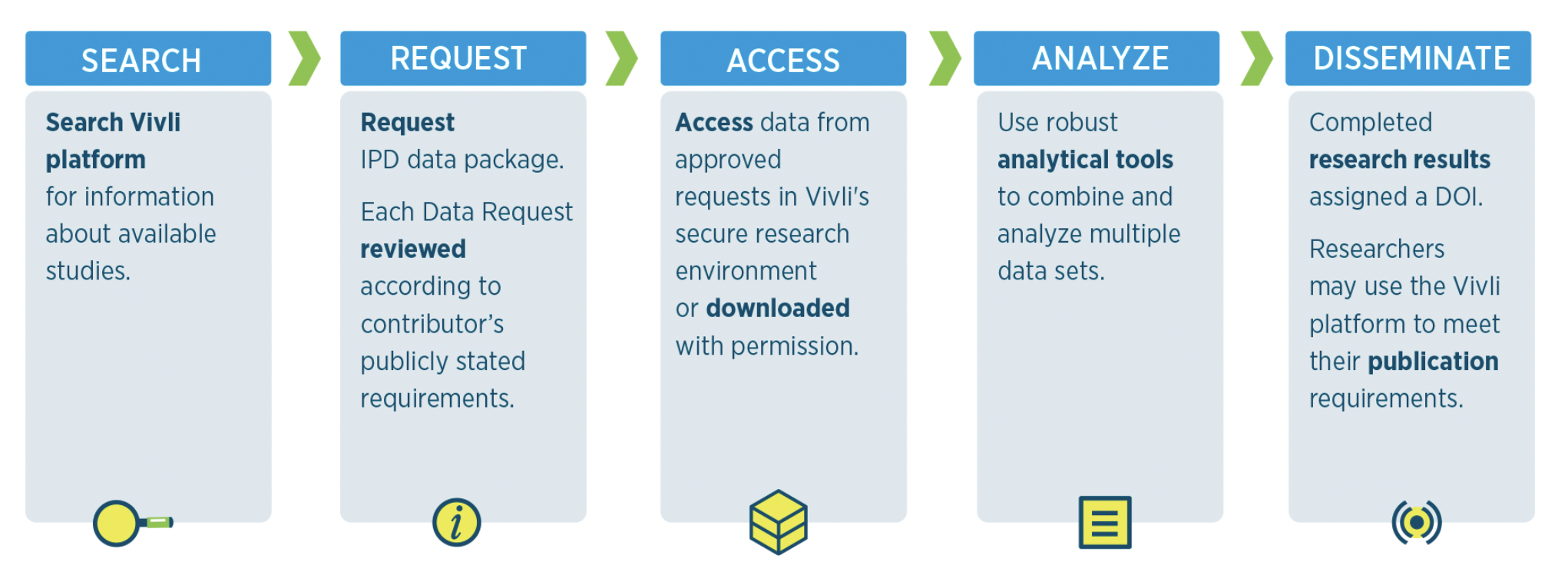
Data should be made available as soon as possible or the acceptance for publication of the main findings from the final dataset but the latest date is the end of the award. Data sharing can be done through a variety of mechanisms, including NIH domain repositories or NIH generalist repositories. These can be open-access or controlled-access systems. One such controlled-access repository recognized by the NIH is Vivli, a generalist repository for sharing of clinical data for human research studies.
As a condition of their grant application, investigators are now required to prospectively plan for management of their data and preparing it for re-use, submit a data management and sharing plan (DMSP), and comply with the drafted plan. The NIH Data Sharing Policy (DSMP) encourages investigators to share their data in order to maximize the value of NIH research funds. But what exactly is a DMSP and how do you draft one for submission to the Vivli Repository? The DMSP is a set of principles and guidelines that outline the requirements for sharing data generated by NIH-funded research. It includes 6 major elements that were selected to ensure that the data is shared as widely and promptly as possible, to maximize the scientific and public health value of the research, while protecting participant privacy and confidentiality. To fill out the DMSP, decisions should be made about the choice of repository, how long the repository will hold/archive the data, whether special tools/software will be provided to access the data, whether consensus data standards apply or exist, whether controlled access will be required and the oversight management details.
To help investigators on their journey to fulfilling the NIH data sharing policy, we have created a list of all the other resources available on our website, including the DMSP template guidance and budget guidance specific to using Vivli to help you navigate the process. Vivli has a step-by-step guide to understanding each of these elements and items to consider when developing a DMSP. We also have a customizable DMSP exemplary language available for download and adaptation, which includes sample text as well as guidance on preparing and submitting a budget as part of the DMSP.
Fill out the form below to access all the DMPS Guidance provided by Vivli.
Vivli has recently released new features timed to the NIH policy launch including: branded portals for research programs / institutions; academic credit; streamlined process for data sharing and reporting for institutions.
In summary, the NIH encourages data sharing as part of its mission to advance biomedical research and to promote collaboration among scientists. Vivli is a non-profit organization that provides a platform recognized by the NIH for funded researchers to share and access anonymized clinical trial data in a secure and compliant way.




 Vivli announced that its Board of Directors has promoted Rebecca Li to the position of CEO. Li previously held the position of Executive Director and has been with the Vivli since its founding when it launched as a project from the MRCT Center of the Brigham and Women’s Hospital and Harvard.
Vivli announced that its Board of Directors has promoted Rebecca Li to the position of CEO. Li previously held the position of Executive Director and has been with the Vivli since its founding when it launched as a project from the MRCT Center of the Brigham and Women’s Hospital and Harvard.






