“Vivli is delighted to have Stanford join with our other academic members to provide a simple, secure way for its researchers to meet the new NIH data sharing policy,” said Rebecca Li, Vivli Chief Executive Officer. “We look forward to working with the Stanford research community to make the data from clinical trials accessible to other researchers to continue to drive forward science.”
Category: News
Senate confirms former Vivli External Advisory Committee member as Director of the National Institutes of Health
Vivli enthusiastically congratulates Dr. Monica Bertagnolli on her recent confirmation as Director of the National Institutes of Health.
Dr. Bertagnolli will become the second woman to lead the NIH on a permanent basis. Previously, Bertagnolli served as Director of the National Cancer Institute and has served as the Richard E. Wilson Professor of Surgery in the field of surgical oncology at Harvard Medical School, a surgeon at Brigham and Women’s Hospital, and a member of the Gastrointestinal Cancer Treatment and Sarcoma Centers at Dana-Farber Cancer Institute. Dr. Bertagnolli has also served as a long-time member on the Vivli External Advisory Committee.
“We are thrilled to hear of Dr. Monica Bertagnolli’s confirmation to such an impactful role. We wish her every success and look forward to witnessing her continued achievements,” said Rebecca Li, CEO.
Keynote speakers: Monica Bertagnolli, the NCI Director, and Robert Califf, FDA Commissioner, to present at the Vivli meeting at the National Academy of Sciences in November.

Vivli is delighted to announce the addition of two keynote speakers to the agenda for our upcoming event, “Shaping the next 10 years in Data Sharing.” Dr. Robert Califf, FDA Commissioner, will address the meeting as the morning keynote speaker; and Dr. Monica Bertagnolli, Director of the US National Cancer Institute (NCI), will join as the lunchtime keynote speaker on Thursday, November 16th.
“Dr. Bertagnolli and Dr. Califf have extensively advocated for data sharing and we eagerly anticipate their insights next month on this important topic,” said CEO Rebecca Li. “We are thrilled they will be joining us.”
Bertagnolli and Califf will join a range of distinguished presenters and participants including researchers, data contributors, publishers, patient advocates, funders, and other interested stakeholders gathering to reflect on the gains made in the past 10 years of data sharing and set new goals and plans for the future.
Dr. Bertagnolli’s lunchtime keynote presentation will be available to those participants attending in person. To be part of this conversation please make a plan to join us in Washington, DC on November 16. Registration is free, but spaces are limited!
Dr. Bertagnolli joined the National Cancer Institute as its director in 2022. Prior to that she served as the Richard E. Wilson Professor of Surgery in the field of surgical oncology at Harvard Medical School, a surgeon at Brigham and Women’s Hospital, and a member of the Gastrointestinal Cancer Treatment and Sarcoma Centers at Dana-Farber Cancer Institute. She has also contributed to multiple initiatives focused on transforming the data infrastructure for clinical research.
Dr. Robert M. Califf was confirmed as the 25th Commissioner of Food and Drugs in 2022. He also served in 2016 as the 22nd Commissioner, and immediately prior to that as the FDA’s Deputy Commissioner for Medical Products and Tobacco. He has spent a good portion of his career affiliated with Duke University, where he served as a professor of medicine and vice chancellor for clinical and translational research, director of the Duke Translational Medicine Institute, and was the founding director of the Duke Clinical Research Institute.
AMR Data Challenge Grand Prize Winners are leveraging the power of AI to combat antimicrobial resistance more effectively
 The World Health Organization (WHO) has identified Antimicrobial Resistance (AMR) as one of the top 10 global health threats facing humanity. Projections warn that antimicrobial-resistant infections have the potential to become the leading cause of death by 2050.
The World Health Organization (WHO) has identified Antimicrobial Resistance (AMR) as one of the top 10 global health threats facing humanity. Projections warn that antimicrobial-resistant infections have the potential to become the leading cause of death by 2050.
Recognizing the need for action on this pressing public health issue, Vivli joined forces with Wellcome in 2022 to launch the AMR Register. This innovative resource houses a growing collection of datasets shared by industry partners, offering consolidated access to surveillance data collected on dozens of antimicrobial interventions.
To raise awareness and encourage reutilization of this wealth of data, Wellcome funded the launch of the AMR Data Challenge in April 2023. The event offered a unique opportunity for multidisciplinary teams to access and leverage high-quality AMR surveillance data, and 56 teams from 28 countries submitted project proposals. The participating teams submitted a wide range of innovative proposals, making use of datasets contributed by GSK, Johnson & Johnson, Paratek, Pfizer, Shinogi, and Venatorx.
Submitted proposals were assessed by a judging panel of international experts, who selected six outstanding proposals for recognition as winners of the AMR Surveillance Open Data Reuse Data Challenge. The team that received the Grand Prize was led by Dr. Fredrick Mutisya, Health Data Scientist & Medical Doctor of Narok County, Kenya, and Dr. Rachael Kanguha, Pediatrician, Chuka County Referral Hospital, Kenya.
Their groundbreaking work involved training machine learning models on the Pfizer ATLAS datasets and the development of a novel artificial intelligence web application capable of predicting antibacterial/antifungal susceptibility. Their proposal notes that traditional methods of prediction have proved insufficiently dynamic to cope with the growing amount of genomic data available, or to effectively monitor and predict trends in antimicrobial resistance, leaving gaps in researchers’ understanding and ability to respond. Their goal is to showcase the best predictive model in order to enable proactive measures and early detection of emerging resistance patterns, and provide a model for ethically and effectively integrating AI into an evidence-based epidemiology approach.
Dr. Mutisya expressed his team’s commitment to AMR and highlighted the importance of providing equitable data accessibility to scientists from his region:
“Our team feels incredibly privileged to have participated in such a meaningful data challenge. Winning the grand prize not only fills us with a profound sense of fulfillment but also ignites a stronger motivation within us to continue seeking solutions for global issues, especially in combating antimicrobial resistance,” he said. “We are deeply grateful to Vivli for providing a platform that facilitates data accessibility. This is particularly significant for scientists like us hailing from the Global South, where opportunities like these are often scarce.”
Five other teams, including scientists from Australia, China, France, India, Spain, the United Kingdom, and the United States, were recognized by the judging panel for proposals which demonstrated notable impact and innovation. A complete list of the winning proposals and finalists is available on the Vivli AMR platform.
Members of the judging panel commented favorably on the excellent quality of the proposals submitted, the innovative approaches used, and creative solutions developed. One of the judges, Professor Marc Mendelson, also noted the importance of open access to data, calling it “a fundamental key to driving innovation towards a better understanding of AMR and the mitigation of this global health crisis.”
The Challenge is over for 2023, but the work of fighting AMR goes on. If you are interested in accelerating research and tackling a global public health challenge at the same time, explore Vivli’s AMR surveillance data sharing platform and find out how you can request access to data.
Awardees Announced for the Vivli AMR Surveillance Open Data Re-Use Data Challenge, funded by Wellcome
Awardees Announced for the Vivli AMR Surveillance Open Data Re-Use Data Challenge, funded by Wellcome
Vivli is pleased to announce the awardees of the Vivli Antimicrobial Resistance (AMR) Surveillance Open Data Re-Use Data Challenge. This initiative comes at a crucial juncture, with the World Health Organization (WHO) identifying Antimicrobial Resistance as one of the top 10 global health threats facing humanity. Alarmingly, antimicrobial-resistant infections have the potential to become the leading cause of death worldwide by 2050. In response to this pressing issue, Vivli and Wellcome joined forces in mid-2022 to launch the AMR Register, a novel platform featuring industry datasets, consolidating surveillance data for the benefit of researchers.
The AMR Data Challenge, funded by Wellcome, was launched in April 2023, as a catalyst for innovation and support for the inventive reutilization of the wealth of surveillance data available within the AMR Register.
“The AMR data challenge not only reflects the extensive interest but also underscores the significance of making AMR data readily accessible to investigators. Data serves as a catalyst for innovative approaches, which are essential in addressing the global AMR challenge,” said Arjun Srinivasan, MD. CAPT, USPS, Deputy Director for Program Improvement Division of Healthcare Quality Promotion, CDC.
A total of 56 teams from 28 different countries participated in the AMR Data Challenge. This event served as a unique platform for multidisciplinary teams to leverage high-quality industry AMR surveillance data, proposing groundbreaking advancements and tools for use in AMR surveillance. The Challenge culminated in the recognition of six outstanding winners for the AMR Surveillance Open Data Re-Use Data Challenge.
The Grand Prize was awarded to Dr. Fredrick Mutisya, Health Data Scientist & Medical Doctor of Narok County, Kenya, and Dr. Rachael Kanguha, Pediatrician, Chuka County Referral Hospital, Kenya. Their groundbreaking work involved training machine learning models on the Pfizer ATLAS datasets and the development of a novel artificial intelligence web application capable of predicting antibacterial/antifungal susceptibility. Dr. Mutisya expressed his team’s commitment to AMR and highlighted the importance of providing equitable data accessibility to scientists from his region,
“Our team feels incredibly privileged to have participated in such a meaningful data challenge. Winning the grand prize not only fills us with a profound sense of fulfilment but also ignites a stronger motivation within us to continue seeking solutions for global issues, especially in combating antimicrobial resistance,” he said. “We are deeply grateful to Vivli for providing a platform that facilitates data accessibility. This is particularly significant for scientists like us hailing from the Global South, where opportunities like these are often scarce.”
Other notable awardees and their project titles include:
- Impact Award Winner: Quentin Leclerc, Institut Pasteur, “Stronger together? Potential and limitations of combining industry datasets to fill in global AMR surveillance gaps.”
- Impact Award Winner: Yanhong Jessika Hu, Murdoch Children’s Research Institute, “Global Geographic Patterns and Trends of WHO Priority Pathogens and AWaRe Antibiotic Resistance Among Children: amrinkids.com.”
- Innovation Award: Robert Beardmore, University of Exeter, “Are antibiotic breakpoints globally consistent, and does it matter if not?”
- Innovation Award Winner: Shraddha Karve, Ashoka University, “Novel approach to antibiogram analysis: looking at the composite resistance phenotype.”
- Innovation Award Runner-up: Jacob Wildfire, LSHTM/SGUL, “Analysis of variations in minimum inhibitory concentration distributions by patient group.”
Data contributed by GSK, Johnson & Johnson, Paratek, Pfizer, Shionogi, and Venatorx was made accessible through the AMR Register, significantly enhancing the impact of the Challenge.
Prof. Marc Mendelson, Chair of the Vivli AMR Scientific Advisory Board, Professor of Infectious Diseases and Head of the Division of Infectious Diseases & HIV Medicine, Groote Schuur Hospital, University of Cape Town noted the exceptional quality of the Challenge applications,
“The quality of applications for the Vivli AMR surveillance Open Re-use Challenge was excellent and it is particularly exciting to see the innovative approaches used,” he said. “Ensuring open access to data across the spectrum of private and public sources is a fundamental key to driving innovation towards a better understanding of AMR and the mitigation of this global health crisis.”
Patricia Bradford, PhD, Antimicrobial Development Specialist and a member of the Judging panel spoke of the innovation of the solutions and their impact, “It was exciting to see the creativity of the various teams with regards to novel uses for the susceptibility data generated by the pharmaceutical industry. Our hope is that these efforts will better enable patient care and foster antimicrobial stewardship on a local level.”
Alisa Serio, PhD, Executive Director of Microbiology and Nonclinical Development at Paratek Pharmaceuticals Inc. was impressed by the innovative approaches taken by the participating teams and noted, “The outputs of this challenge are exactly what the Vivli AMR initiative was set up for, specifically to openly share surveillance data for researchers to investigate a myriad of questions in AMR to help further understanding, decision-making and policy changes worldwide.”
For more details and to view the winning teams’ solutions, please visit https://amr.vivli.org/data-challenge/finalist-and-award-winning-solutions.
Contact: Catherine D’Arcy, Rebecca Li
About Vivli
Vivli is a non-profit organization working to advance human health through the insights and discoveries gained by sharing and analyzing data. Data sharing initiatives include the AMR Register for AMR surveillance data and the Vivli Platform for clinical trial data. Vivli acts as a neutral broker between data contributor and data user and the wider data sharing community. For more information, visit www.vivli.org and follow us on LinkedIn and Twitter @VivliCenter.
Vivli Researcher Spotlight: Dr. Yizhe Xu on analyzing clinical trial data to inform development of machine learning tools
 Yizhe Xu is a Postdoctoral Researcher at Stanford Center for Biomedical Informatics Research, Stanford University. Dr. Xu’s team submitted a research proposal to access Vivli to conduct analysis relevant to their topic, “Applying machine learning tools to personalize dabigatran treatment decisions”. The team’s completed research has been presented to the research community at conferences and in publications including the Journal of Biomedical Informatics. She sat down with Vivli to tell us more about accessing individual participant data to advance her research, and the potential for machine learning tools to support more accurate estimation and evaluation of heterogeneous treatment effects.
Yizhe Xu is a Postdoctoral Researcher at Stanford Center for Biomedical Informatics Research, Stanford University. Dr. Xu’s team submitted a research proposal to access Vivli to conduct analysis relevant to their topic, “Applying machine learning tools to personalize dabigatran treatment decisions”. The team’s completed research has been presented to the research community at conferences and in publications including the Journal of Biomedical Informatics. She sat down with Vivli to tell us more about accessing individual participant data to advance her research, and the potential for machine learning tools to support more accurate estimation and evaluation of heterogeneous treatment effects.
Tell us more about your research. What is the current state of management in your public disclosure topic?
Our final paper has been published in the Journal of Biomedical Informatics as of July 2023.
What led you to want to research this topic?
First of all, treatment effect heterogeneity is an important question that informs clinical decision making given the fact that treatment effects often vary across patients. Thus, accurate estimation of individual treatment effects helps to tailor treatment to patient characteristics and maximizes their benefits. However, it has been realized by a wide group of researchers that estimating treatment heterogeneity is challenging, so we summarized the best practices and advanced methodologies and showed a case study on how to carefully estimate heterogeneous treatment effects using the RE-LY and RELY-ABLE trials.
What difference do you hope your research might make, either in the field or for patients? How has it moved forward the treatment of patients?
We hope our case study provides clear instructions and serves as a concrete example for clinical researchers, and that by following our suggestions, they will be able to avoid possible false discoveries of treatment heterogeneity and prevent misleading findings. The improvement of research quality will directly benefit everyday clinical care in the sense that patients will truly benefit from personalized treatment selection if there is treatment heterogeneity and can be estimated reasonably well. On the other hand, we can save the clinicians’ time and efforts on considering personalized treatment when the treatment effect is essentially uniform across patients.
How could your findings be used in future clinical trials in your disease area?
The statistical methods we have summarized and the guidance we provide on how to select a method and evaluate the model performance can be applied to clinical trials in any disease area. However, for observational studies, practitioners need to consider adjusting for confounding, for instance, using methods such as propensity score matching or weighting.
How did the data you accessed through Vivli help you in answering your research question?
Very well. The RE-LY trial enables a case study for us to demonstrate the principled approach we proposed for estimating heterogeneous treatment effect in a real study. The RE-LY study has a large data size, and the fact that it is an RCT helps to simplify the task of treatment effect estimation.
What was your experience like in the process of requesting data using the Vivli platform?
It was an okay experience – we had some difficulties in resolving issues related to the DUA, which made us wait for quite a while, but we were able to get the access eventually.
Would you use the Vivli platform again? Would you recommend Vivli to others? What improvements would you recommend?
Yes, especially if I think some of the unique data sources on the Vivli platform will help to answer particular research questions of my interest. I will also recommend Vivli to others for the same reason. I would recommend simplifying the data requesting process to shorten the waiting time, as well as expanding user autonomy – particularly that users do not need to make a request every time they want to export results.
What advice would you give to other researchers about doing this kind of analysis?
We encourage researchers to understand their data first, then select the most suitable statistical approaches based on that knowledge. After that, we suggest interpreting findings based on the results from multiple estimators that are weighted by their performance, which is evaluated using several different metrics. Please also see our paper for our detailed recommendations.
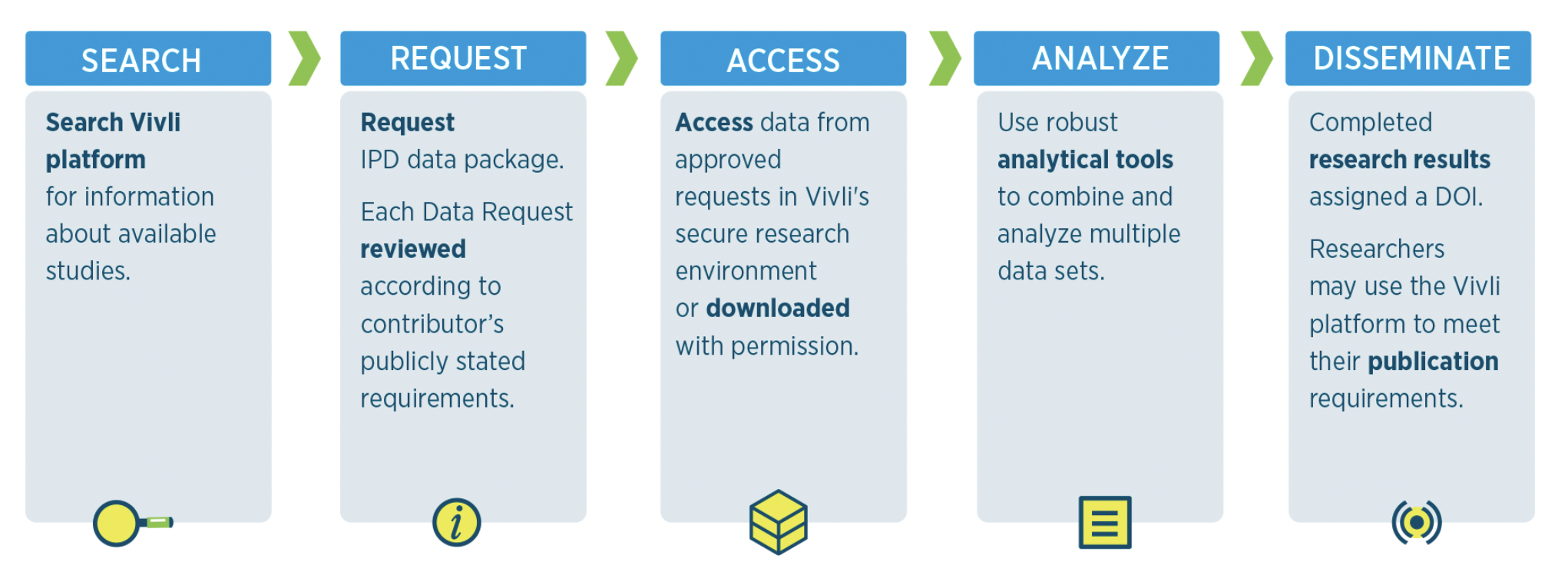
Interested in finding out more about how access to Vivli’s data repository can help advance your research? Find out more about how to search and request data.
Vivli launches Portals to support more effective data sharing and data reuse
Vivli is delighted to announce the launch of our new Portals feature, designed to highlight therapeutic areas of interest to the research community. One of our first portals focuses on available data relevant to HIV/AIDS, a priority area of research focus for NIH.
We’ve designed Portals to make data from HIV/AIDS clinical trials more visible and discoverable. Vivli’s repository of data is built on nearly 7,000 research studies across a wide range of research areas, and includes a growing number being shared by individual researchers. This data repository provides a valuable resource for researchers to both share and access data that can be used to accelerate the progress of scientific research.
Are you an HIV/AIDS researcher? We’d love to hear from you, whether you’ve got data to share or are interested in exploring our data resources to request. Explore our new HIV/AIDS Portal and find out more about how Vivli can support your research.
This is funded in whole or in part with Federal funds from the Office of AIDS Research, National Institutes of Health, 1OT2DB000003-01, awarded to Vivli.
Vivli celebrates publication of 200th public disclosure

Vivli is delighted to announce publication of the 200th public disclosure resulting from the research team’s work with data from the Vivli platform.
Rebecca Li, the Chief Executive Officer of Vivli, congratulates all the research teams who have utilized data from the Vivli platform to advance health research through the re-use of valuable clinical trial data. She also acknowledges the organizations, individuals, and thousands of trial participants who have generously shared their data, making this milestone possible.
The Vivli repository houses data from nearly 7,000 trials, representing the contributions of 1.8 million clinical trial participants. On average, Vivli public disclosures are cited approximately 2.2 times per publication and appear in a wide range of highly-ranked academic journals.
For more information about how to share and re-use data on the Vivli platform, please visit our Resources page.
Vivli Senior Advisor speaks at CDISC 2023 Japan Interchange Program
Vivli Senior Advisor Azusa Tsukida spoke at Clinical Data Interchange Standards Consortium (CDISC) 2023 Japan Interchange Program on July 10.
Tsukida presented during the session on ‘Real World Data & Regulatory Presentations/Perspectives’. Her talk focused on the benefits of data sharing, using case studies from data contributors who are sharing high-quality data via the Vivli platform to enable access to researchers worldwide and contribute to scientific discovery.
CDISC works to develop and advance data standards to support transforming incompatible formats, inconsistent methodologies, and diverse perspectives into a coherent framework for generating clinical research data that is accessible, interoperable, and reusable. More than 80% of the data available in Vivli is formatted in the CDISC-SDTM standard.
Find out more about how you can request data from Vivli’s repository and help accelerate the progress of health research.
Vivli Researcher Spotlight: Dr. Fasihul Khan on the potential for biomarkers to predict outcomes for people with pulmonary fibrosis

Fasihul Khan, M.D., Ph.D., is a consultant at Glenfield Hospital, University Hospitals of Leicester NHS UK. Dr. Khan’s team submitted a research proposal to access Vivli to conduct analysis relevant to their topic, “A systematic review and individual patient data meta-analysis of physiological biomarkers in idiopathic pulmonary fibrosis”. The team’s completed research has been presented to the research community at conferences and in publications including American Journal of Respiratory and Critical Care Medicine. He sat down with Vivli to tell us more about accessing individual participant data to advance his research, and the potential for biomarkers to predict outcomes for people with pulmonary fibrosis.
Please tell us more about your research – what led you to want to research this particular topic?
So my area of interest is pulmonary fibrosis, which is a condition causing scarring of the lungs. Pulmonary fibrosis is a relatively rare condition, and therefore the number of studies in this area are limited, although expanding rapidly. I was keen to synthesize some of the existing information that was already available. I wanted to perform a systematic review, specifically looking to see whether there are blood biomarkers that can predict outcomes in patients with diagnosed pulmonary fibrosis. When I started searching the literature, it very quickly became apparent that there were several published studies, but actually the data and the way the studies were reported were very heterogeneous. Individually the studies yielded inconsistent results, utilized data-dependent thresholds, and frequently did not adjust for confounders. Therefore, I sought individual participant data which helped overcome these limitations and enabled robust data analyses to be performed leading to reliable conclusions.
Could you talk about what it was like to work across multiple data-sharing platforms; how did you handle that?
This was not straightforward! I created summary estimates from each study separately on the different platforms in Vivli and in CSDR, then imported them manually onto my own database. I then used additional software to pool the summary estimates. Having the data all in one place would have saved me a lot of time and stress!
Not a lot of researchers have the perseverance to do what you did. What advice would you give to researchers before they start off? Things you wish you’d known before you started?
I think it’s important to consider the project as a whole. It is highly likely the process will take much longer than you think, and that’s not necessarily any individual or organization’s fault. You need to have a clear understanding with contingency plans for each stage, and give yourself plenty of time! Be clear about your research question, and whether individual participant data are likely to improve your research, before committing to the additional effort. Speak to others who have been through the process of acquiring individual participant data, and your institution to understand timescales for data sharing agreements as these are likely to be time consuming and potential limiting factors.
Once you were able to access the individual patient data, were you able to get past the reporting limitations and find what you needed?
Absolutely; once we had the raw data, we were able to perform our analysis and produce some very meaningful results, which we have subsequently published in two journals. The first was a blood biomarker paper in the European Respiratory Journal which was the first blood biomarker study in pulmonary fibrosis to utilize this approach, and provides robust estimates of the association between matrix-metalloproteinase 7 and disease progression.
The second paper was published in the American Journal of Respiratory Critical Care Medicine. In this paper, we looked at change in FVC which is a lung function measurement used to assess progression in pulmonary fibrosis. All interventional clinical trials measure FVC as an endpoint – typically at 12 months, but patients have additional FVC measurements at baseline, 3, and 6 months. The purpose of our research was to evaluate whether short term changes in FVC i.e. over three-months, are associated with overall mortality. In other words, can we shorten clinical trials by finding an earlier signal than the 12 months FVC change that is currently accepted by regulators. Since the association between short term FVC change and mortality was not reported in any clinical study, we needed the individual participant data to model this association. Indeed, we were able to find that three-month FVC change is associated with mortality, and perhaps more importantly a treatment effect could be observed between treatment and placebo arms at three-months. The findings of this study have been well received by the research community, and have already been adopted into the design of an adaptive trial in IPF. Lots of hard work, but worth it as the results are likely to generate further research which ultimately will hopefully impact patients in a positive manner!

