
This event was held at Harvard Faculty Club in Cambridge, MA, on Friday, November 15, for the 2024 Vivli Annual Meeting, co-hosted with the MRCT Center of Brigham and Women’s Hospital and Harvard. The meeting focuses on Innovations in Data Sharing and features a keynote address from Steffen Thirstrup, CMO of the EMA. He speaks on the European Health Data Space.
RECORDINGS SPEAKER BIOGRAPHIES
Session topics focus on the following areas:
- Keynote address: Steffen Thirstrup, EMA on European Health Data Space
- Panel discussion on European Health Data Space
- Health Information Exchanges
- Risks and Opportunities in AI and data sharing
Agenda

Keynote: Steffen Thirstrup, CMO, European Medicines Agency (EMA)
Looking to the future: The opportunities for data sharing and the European Health Data Space
RECORDING

Panel Discussion: The Opportunities for data sharing and the European Health Data Space (EHDS)
Following on from the keynote address, panelists discuss the potential of the EHDS.
Moderator: Rebecca Li, CEO and Co-founder, Vivli
- David Leventhal, Data Sharing & Disclosure Lead, Pfizer
- David McAllister, Professor of Clinical Epidemiology and Medical Informatics, University of Glasgow
- Steffen Thirstrup, CMO, EMA
- Aneta Tyszkiewicz, Director, Digital & Data, EFPIA
RECORDING

Panel Discussion: Use of Health Information Exchange (HIE) data for research: legal and ethical challenges
The panel discusses challenges related to the use of HIEs and other U.S. data sharing frameworks for research purposes, including research recruitment, retrospective studies, and more.
Moderator: Barbara Bierer, Faculty Director, MRCT and Vivli co-founder
- Jill De Graff, VP of Regulatory, b.well Connected Health
- Irene Koch, Executive Vice President and Chief Legal Officer, Hospital for Special Surgery, New York City
- David Peloquin, Partner, Ropes & Gray
RECORDING

Panel Discussion: Risks and Opportunities in AI and data sharing
Panelists discuss AI’s risks and opportunities for sharing and re-using data today and in the coming years.
Moderator: Ida Sim, Professor of Medicine and Computational Precision Health, University of California San Francisco; Vivli co-founder
- Karla Childers, Head, Bioethics-based Science & Technology Policy, Johson & Johnson
- Dawei Lin, Associate Director for Bioinformatics & Senior Advisor to the Director at DAIT, NIAID, NIH
- Subha Madhavan, Vice President & Head of AI/ML, Quantitative & Digital Sciences, Research and Development, Pfizer and Vivli Board member
- David Peloquin, Partner, Ropes & Gray
RECORDING





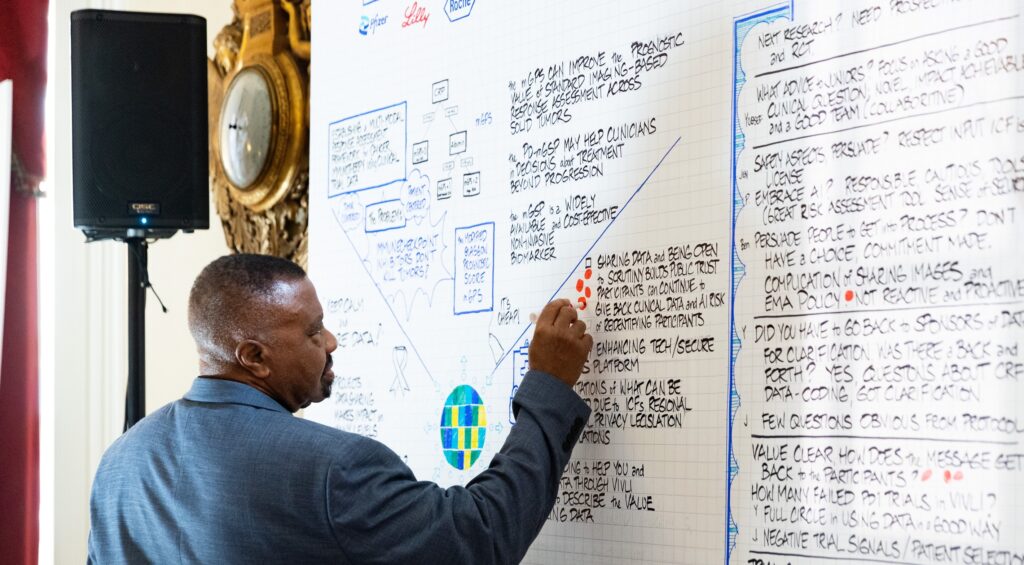




 The 2025 Vivli Annual Meeting was held at Loeb House at Harvard University in Cambridge, MA, and virtually on Thursday, October 23.
The 2025 Vivli Annual Meeting was held at Loeb House at Harvard University in Cambridge, MA, and virtually on Thursday, October 23.
 Are you a researcher who has analyzed data on Vivli? Or has your research team already analyzed data in the Vivli platform but is looking for more efficient approaches?
Are you a researcher who has analyzed data on Vivli? Or has your research team already analyzed data in the Vivli platform but is looking for more efficient approaches?





