 Yizhe Xu is a Postdoctoral Researcher at Stanford Center for Biomedical Informatics Research, Stanford University. Dr. Xu’s team submitted a research proposal to access Vivli to conduct analysis relevant to their topic, “Applying machine learning tools to personalize dabigatran treatment decisions”. The team’s completed research has been presented to the research community at conferences and in publications including the Journal of Biomedical Informatics. She sat down with Vivli to tell us more about accessing individual participant data to advance her research, and the potential for machine learning tools to support more accurate estimation and evaluation of heterogeneous treatment effects.
Yizhe Xu is a Postdoctoral Researcher at Stanford Center for Biomedical Informatics Research, Stanford University. Dr. Xu’s team submitted a research proposal to access Vivli to conduct analysis relevant to their topic, “Applying machine learning tools to personalize dabigatran treatment decisions”. The team’s completed research has been presented to the research community at conferences and in publications including the Journal of Biomedical Informatics. She sat down with Vivli to tell us more about accessing individual participant data to advance her research, and the potential for machine learning tools to support more accurate estimation and evaluation of heterogeneous treatment effects.
Tell us more about your research. What is the current state of management in your public disclosure topic?
Our final paper has been published in the Journal of Biomedical Informatics as of July 2023.
What led you to want to research this topic?
First of all, treatment effect heterogeneity is an important question that informs clinical decision making given the fact that treatment effects often vary across patients. Thus, accurate estimation of individual treatment effects helps to tailor treatment to patient characteristics and maximizes their benefits. However, it has been realized by a wide group of researchers that estimating treatment heterogeneity is challenging, so we summarized the best practices and advanced methodologies and showed a case study on how to carefully estimate heterogeneous treatment effects using the RE-LY and RELY-ABLE trials.
What difference do you hope your research might make, either in the field or for patients? How has it moved forward the treatment of patients?
We hope our case study provides clear instructions and serves as a concrete example for clinical researchers, and that by following our suggestions, they will be able to avoid possible false discoveries of treatment heterogeneity and prevent misleading findings. The improvement of research quality will directly benefit everyday clinical care in the sense that patients will truly benefit from personalized treatment selection if there is treatment heterogeneity and can be estimated reasonably well. On the other hand, we can save the clinicians’ time and efforts on considering personalized treatment when the treatment effect is essentially uniform across patients.
How could your findings be used in future clinical trials in your disease area?
The statistical methods we have summarized and the guidance we provide on how to select a method and evaluate the model performance can be applied to clinical trials in any disease area. However, for observational studies, practitioners need to consider adjusting for confounding, for instance, using methods such as propensity score matching or weighting.
How did the data you accessed through Vivli help you in answering your research question?
Very well. The RE-LY trial enables a case study for us to demonstrate the principled approach we proposed for estimating heterogeneous treatment effect in a real study. The RE-LY study has a large data size, and the fact that it is an RCT helps to simplify the task of treatment effect estimation.
What was your experience like in the process of requesting data using the Vivli platform?
It was an okay experience – we had some difficulties in resolving issues related to the DUA, which made us wait for quite a while, but we were able to get the access eventually.
Would you use the Vivli platform again? Would you recommend Vivli to others? What improvements would you recommend?
Yes, especially if I think some of the unique data sources on the Vivli platform will help to answer particular research questions of my interest. I will also recommend Vivli to others for the same reason. I would recommend simplifying the data requesting process to shorten the waiting time, as well as expanding user autonomy – particularly that users do not need to make a request every time they want to export results.
What advice would you give to other researchers about doing this kind of analysis?
We encourage researchers to understand their data first, then select the most suitable statistical approaches based on that knowledge. After that, we suggest interpreting findings based on the results from multiple estimators that are weighted by their performance, which is evaluated using several different metrics. Please also see our paper for our detailed recommendations.
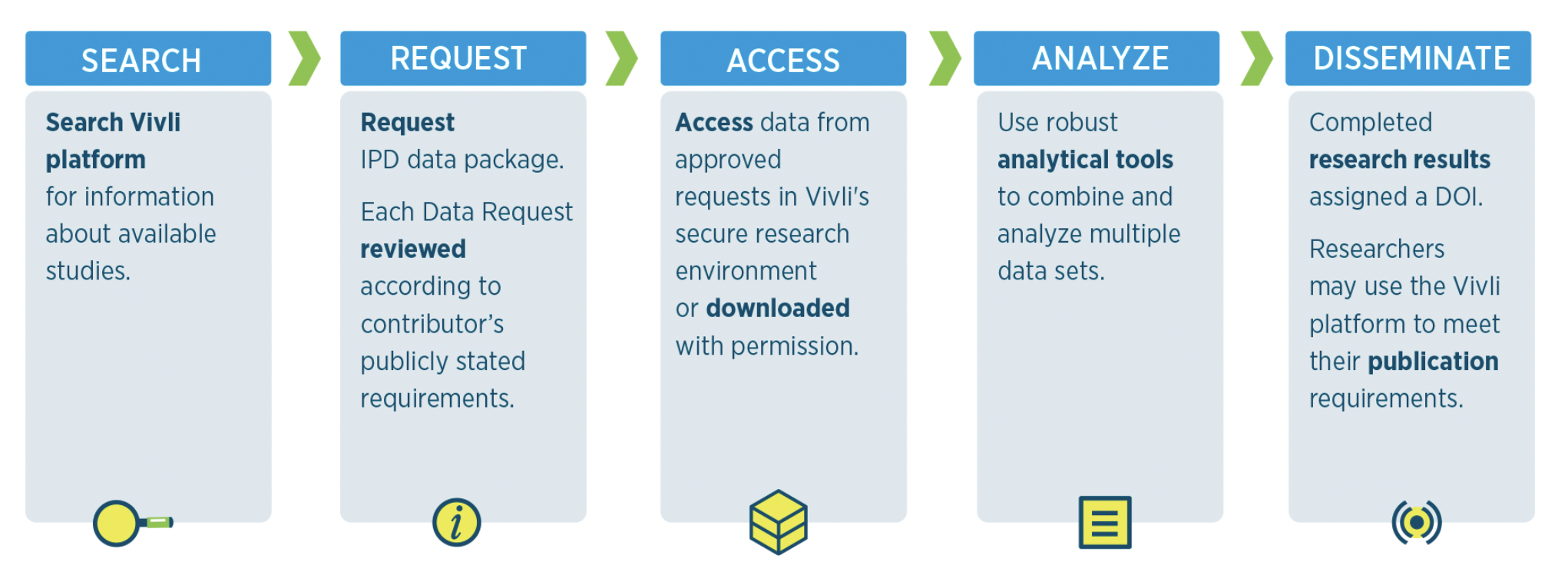
Interested in finding out more about how access to Vivli’s data repository can help advance your research? Find out more about how to search and request data.




 Vivli announced that its Board of Directors has promoted Rebecca Li to the position of CEO. Li previously held the position of Executive Director and has been with the Vivli since its founding when it launched as a project from the MRCT Center of the Brigham and Women’s Hospital and Harvard.
Vivli announced that its Board of Directors has promoted Rebecca Li to the position of CEO. Li previously held the position of Executive Director and has been with the Vivli since its founding when it launched as a project from the MRCT Center of the Brigham and Women’s Hospital and Harvard.